Abstract
Background:
The incidence of venous thromboembolism (VTE) is much higher in patients with cancers than those without. Evidence to support cancer-associated VTE as a distinct pathological entity has mounted in recent years. Previous study using a national insurance based database reported that cancer-associated VTE incidence in Taiwan from 1997 to 2005 was only 1.85 per 1000-patient-year, which is much lower than reports in western countries and our clinical observation. However, this approach might have had limitations in identifying all VTE cases and had no access to many clinical parameters. To address this issue using a more detailed approach, we initiated an investigation locally with a comprehensive review of medical records and deeper profiling of demographic and laboratory parameters in the hope of gaining new insights about the incidence and risk factors for cancer-associated VTE, particularly in those cancers with a higher risk of VTE.
Methods:
Medical records of all adult patients with lung, pancreatic, gastric cancers and lymphoma from January 2011 to December 2013 in National Taiwan University Hospital indexed through the local cancer registry database were retrospectively reviewed. Histology types, treatments, stages, metastases, patients' profiles and laboratory data at diagnosis of cancer were also analyzed. Cancer-associated VTE was defined as the first VTE event, proven by image studies, occurring after the diagnosis of cancer, or within one year before it. To exhaust the identification of all possible VTE patients, the cancer registry database was intersected with pharmacy data, followed by a detailed medical record review to confirm the VTE diagnosis, location and anticoagulants used. The median follow-up was 12.6 months.
Results:
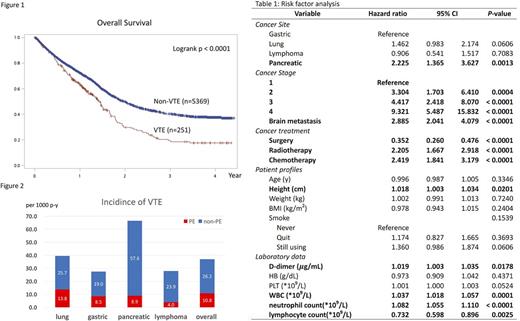
Data was collected from 5620 cancer patients, 3136, 920, 709, and 855 with lung, gastric, pancreatic cancer and lymphoma, respectively. VTE was diagnosed in 251 of them (4.5%). The patients with VTE had significantly shorter overall survival than those without VTE (17.0 months vs. 23.9 months, p < 0.0001, Figure 1). Overall VTE incidence was 37.0 per 1000 patient-year, and 39.5, 27.5, 66.5, and 23.9 per 1000 patient-year for lung, gastric, pancreatic cancer and lymphoma, respectively, as shown in Figure 2. Among VTE patients, upper limb VTE happened in 71 patients (28.3%) and pulmonary embolism (PE) in 75 patients (29.5%). VTE-related risk factors of all patients are demonstrated in Table 1. Brain metastasis, advanced stage, chemotherapy, radiotherapy, higher d-dimer, higher WBC counts and higher neutrophil counts were significantly related to higher VTE risk. The association of surgery with lower VTE risk might be a confounding effect because patients receiving surgery were usually in the earlier stage of the disease and thus had limited VTE risk. Smoking, body mass index (BMI) or weight, age and hemoglobin level were not related to VTE risk. Higher platelet counts had a trend to be associated with higher VTE risk. Interestingly, taller patients had higher risk of VTE than shorter ones, but the difference was significant only for upper limb VTE (1.048 [1.019-1.078]) but not for VTE of other parts (1.006 [0.988-1.024]). The majority of VTE patients (192, 76.5%) received enoxaparin as the main anticoagulation treatment, while 19.5% of patients received a vitamin K antagonist, two patients (0.8%) received rivaroxaban and eight patients (3.2%) did not receive any anticoagulation.
Conclusion
The incidence of cancer-associated VTE among four high-risk cancer types was 37.0 per 1000-patient-year from 2011 to 2013, which was much higher than previous reports in Taiwan. We assumed the difference might be caused by using medical record review rather than diagnosis coding acquisition to identify VTE cases. We could not find a significant association between VTE and age, hemoglobin, or BMI. However, we found that height is associated with risk of upper limb VTE which may need more studies to verify the significance. The majority of cancer-associated VTE patients here were treated with enoxaparin as international guidelines suggested.
Tsai: Celgene International Sàrl: Research Funding. Tien: Celgene International Sàrl: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal